Give the Oxidation Number of Chromium in the Following
Click here to check your answer to Practice Problem 14. The increase in atmospheric and oceanic O 2 content following the GOE led to the widespread oxidation of redox-sensitive metals 1730 with a corresponding increase in Earths mineral diversity.
Solved Give The Oxidation Number Of Chromium In The Chegg Com
Oxidation of organic compounds generally.

. Second we apply the definitions of oxidation and reduction. Chromium plating was once widely used to give steel a polished silvery mirror coating. Chromium is unstable in oxygen it immediately produces a thin oxide layer that is impermeable to oxygen and protects the metal below.
Cr 2 O 3 film on chromium or on chromium steels has poor ionic conductance and thus prevents further oxidation. These functional groups are useful for further reactions. Other articles where oxidation is discussed.
Since the magnesium atom has a 2 oxidation number this means that each chlorine atom must have a -1 oxidation number. The magnetic carbon exhibited high adsorption capacity in CrVI removal applications following a pseudosecond order kinetic model. First we assign oxidation states or numbers to all the atoms in the reaction and determine the elements that are changing oxidation state.
Chromium-plated car and lorry parts such as bumpers were once very common. Algebra I Module 2. Alcohols may be oxidized to give ketones aldehydes and carboxylic acids.
In this module students reconnect with and deepen their understanding of statistics and probability concepts first introduced in Grades 6 7 and 8. The oxidation state or oxidation number is the hypothetical charge of an atom if all of its bonds to different atoms were fully ionicIt describes the degree of oxidation loss of electrons of an atom in a chemical compoundConceptually the oxidation state may be positive negative or zero. Oxidation states if the other atoms in the ion have known oxidation numbers.
The oxidation number of oxygen in a compound is usually 2. If however the oxygen is. Redox reactions are characterized by the actual or formal transfer of electrons between chemical species most often with one species the reducing agent undergoing oxidation losing electrons while another species.
Chromium plating can be used to give a polished mirror finish to steel. The interconversion of iron oxidation states is a mechanism whereby iron participates in electron transfer as well as a mechanism whereby iron can reversibly bind. Values are given for typical oxidation number and coordination.
-2 2 0. In biological systems these oxidation states occur primarily as the ferrous 2 ferric 3 and ferryl 4 states. The maximum adsorption capacity was 88382 mgCrVIgAC at pH 3.
Iron can exist in oxidation states ranging from 2 to 6. 2 chlorine atoms give us a total of -2. Chromium main uses are in alloys such as stainless steel in chrome plating and in metal ceramics.
For example ketones and aldehydes can be used in subsequent Grignard reactions and carboxylic acids can be used for esterification. Script-like ZrC carbides were obtained in the. Cd increases in oxidation state from 0 to 2 and Ni decreases from 4 to 2.
The oxidation number of an alkaline earth metal IIA family in a compound is 2. While fully ionic bonds are not found in nature many bonds exhibit strong ionicity making. The oxide film is protective if it has good adherence is impervious non-volatile non-reactive with atmosphere has similar co- efficient of expansion as the metal if temperature variations are cyclic.
After control of their as-cast microstructures these alloys were subjected at 1100 C to flexural creep and to oxidation in air. Redox reductionoxidation ˈ r ɛ d ɒ k s RED-oks ˈ r iː d ɒ k s REE-doks is a type of chemical reaction in which the oxidation states of atoms are changed. The oxidation number of an alkali metal IA family in a compound is 1.
Determine the oxidation number of the chromium in an unknown salt if electrolysis of a molten sample of this salt for 150 hours with a 100-amp current deposits 971 grams of chromium metal at the cathode. And we ask you to honour and agree to the following terms and conditions when using this Site. Finally oxidation experiments in combination with FT-IR Mssbauer and VSM measurements indicated that the possible Cr6 removal.
Three alloys based on nickel rich in chromium due to their oxidation resistance at high temperature and containing ZrC carbides for their mechanical reinforcing against creep were elaborated by foundry.

Solved Give The Oxidation Number Of Chromium In Each Of The Chegg Com

How To Find The Oxidation Number For Cr In The Cr2o7 2 Ion Dichromate Ion Youtube
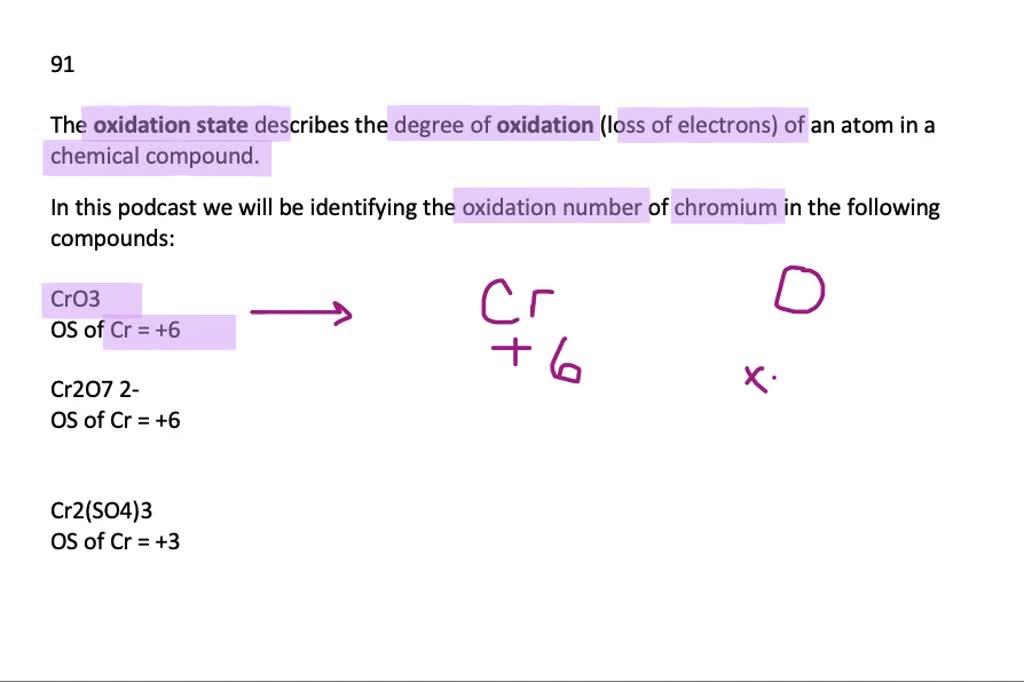
Solved Give The Oxidation Number Of Chromium In Each Of The Following A Mathrm Cro 3 B Mathrm Cr 2 Mathrm O 7 2 C Mathrm Cr 2 Left Mathrm So 4 Right 3
No comments for "Give the Oxidation Number of Chromium in the Following"
Post a Comment